体内研究
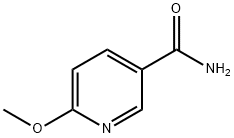
JBSNF-000088 (6-Methoxynicotinamide) (50 mg/kg; oral route of administration for four weeks) shows statistically significant reduction in body weight (%) and leads to a statistically significant reduction in fed blood glucose on day 21.
JBSNF-000088 (50 mg/kg; oral gavage administration; twice daily for four weeks) leads to a statistically significant improvement in oral glucose tolerance on day 28 with glucose tolerance being normalized.
JBSNF-000088 (1 mg/kg; intravenous administration; for 4 hours) results in low plasma clearance of 21 mL/min▪kg and the volume of distribution at steady state of 0.7 L/kg, a very short plasma half-life of 0.5 hours upon intravenous administration.
JBSNF-000088 (10 mg/kg; oral gavage; for 4 hours) results in a Cmax of 3568 ng/mL with a T
max
value of 0.5 hours, indicating rapid absorption in the intestine, and half-life of 0.4 hours by oral gavage. The oral bioavailability is found to be approximately 40%.
Animal Model:
Mice with high fat diet (HFD)-induced obesity
Dosage:
50 mg/kg
Administration:
Oral route of administration for four weeks; oral gavage administration and twice daily for four weeks
Result:
Showed statistically significant reduction in body weight (%) and led to a statistically significant reduction in fed blood glucose on day 21 by oral route of administration.
Led to a statistically significant improvement in oral glucose tolerance on day 28 with glucose tolerance being normalized by oral gavage administration.
Animal Model:
C57BL/6 mice
Dosage:
1 mg/kg (Intravenous administration);10 mg/kg (oral gavage)(Pharmacokinetic Study)
Administration:
Intravenous administration and oral gavage; for 4 hours
Result:
Resulted in low plasma clearance of 21 mL/min▪kg and the volume of distribution at steady state of 0.7 L/kg, a very short plasma half-life of 0.5 h upon intravenous administration.
Resulted in a Cmax of 3568 ng/mL with a T
max
value of 0.5 hours, indicating rapid absorption in the intestine, and half-life of 0.4 hours by oral gavage.