体外研究
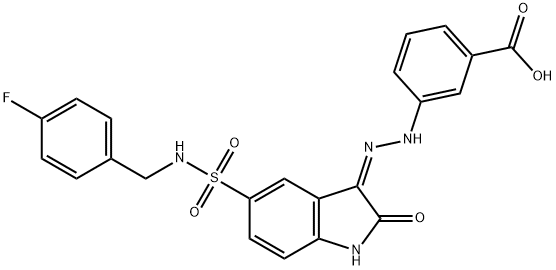
SPI-112 has a polar -NO
2
or a negatively charged -COOH group and has no detectable cellular activity, suggesting that SPI-112 is not cell permeable.
In surface plasmon resonance (SPR) binding assay, SPI-112 displays a 1:1 stoichiometric binding kinetics to SHP2 with a kinetic constant K
D
of 1.30 µM. Enzyme kinetic data obtained with SPI-112 are best fitted with the competitive inhibition model (K
i
of 0.8 µM), suggesting that SPI-112 interacts with the catalytic site of SHP2.